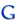Formula to convert N.P.K in to fertilizer quantity
Common fertilizers and their nutrient contents
Table with formula to convert recommended N.P.K in to fertilizer quantity (Kgs):
Name of the Nutrient
|
Formula
|
Fertilizer
|
N
|
2.17
|
Urea
|
N
|
5.00
|
AmSo4
|
N
|
4.00
|
Ammonium Chloride
|
N
|
3.03
|
Ammonium Nitrate
|
N
|
5.56
|
D.A.P {Di Ammonium Phosphate}
|
‘P’ soluble in Water
|
2.17
|
D.A.P {Di Ammonium Phosphate}
|
‘P’ soluble in Water
|
6.25
|
Single super phosphate
|
‘P’ soluble in Acids
|
3.0
|
Di Calcium Phosphate
|
K
|
1.67
|
M.O.P {Muriate of Potash}
|
Example: If you want to give 50 Kgs of ‘N’ in the form of “UREA’, then calculate as :
50 x 2.17 = 108.5 Kgs.
Example II: If you want to give 33 Kgs of ‘N’ and 85 Kgs of ‘P’, through D.A.P then calculate as: For ‘N’ = 33 x 5.56 = 183 Kgs. For ‘P’ = 85 x 2.17 = 185 Kgs.
So, applying 185 Kgs of D.A.P will meet both the N & P requirements of the crop.
Example III: If you want to give 50 Kgs of ‘N’ and 85 Kgs of ‘P’ then calculate as:
‘P’ thro’ D.A.P = 85 x 2.17 = 185 Kgs.
‘N’ quantity in 185 Kgs of D.A.P = 185 / 5.56 = 33 Kgs.
(Total Requirement of N 50 - 33 of N in 185 Kgs of DAP = 17 kgs of deficit ‘N’}
This deficit ‘N’ can be supplemented by adding Urea. (Urea = 17 x 2.17 = 37 Kgs.)
So, we have to mix DAP 185 Kgs and Urea 37 Kgs to meet 50 Kgs of ‘N’ and 85 Kgs of ‘P’ requirements of the crop.